Research Interests
Our major research interests are (1) to uncover fundamental principles of neural circuit function with regard to computation and learning, both under physiological and pathological conditions, and (2) to enable such studies by advancing especially optical methods for studying neuronal network dynamics in the living brain at work ('in vivo'). Ongoing projects include:
Dissecting Neural Circuit Dynamics
Information Processing in Cortical MicrocircuitsUsing a variety of optical methods as well as electrophysiogical techniques we investigate the functional organization of the mammalian neocortex. We are interested in dissecting signal processing within the microcircuits in specific areas (such as primary sensory areas) as well as the signal flow between communicating cortical regions, such as for example sensory, motor, and association areas. We apply two-photon as well as wide-field calcium imaging to measure activity patterns across the various layers of neocortex. Using transgenic mouse lines we aim to dissect the contribution of specific neuronal subtypes, including different types of GABAergic interneurons, as well as of axonal projection pathways. More recently we have also begun to explore in depth subcortical, e.g., deep hippocampal, regions (Pilz et al. 2016). Sensorimotor integration in the whisker systemIn rodents, the facial whiskers ('vibrissae') are a major sensory system, which shows a large one-to-one topographic representation in the primary somatosensory cortex (so-called 'barrel cortex'). To study neural mechanisms underlying tactile discrimination behavior we have established a texture discrimination task for mice (Chen et al., 2013, 2015). Using this task we uncovered that signal routing via divergent pathways is behavior-dependent and that distinct pathways adapt differentially during task learning. In our current research we aim at a deeper understanding of the mechanisms governing information processing, decision-making, short-term memory, and action control when animals perform this task. Computation in neuronal dendritesIndividual neurons are sophisticated signal processing devices that integrate the myriad of synaptic inputs they receive. The dendrites of neocortical pyramidal neurons, for example, contain various voltage-dependent ion channels that produce non-linear signal integration properties (e.g. Waters et al. 2003, Larkum et al. 2007). A long-standing interest of our group is to understand how dendrites translate their synaptic input activity patterns to streams of meaningful, i.e. computationally relevant, output action potentials. We use two-photon calcium imaging as well as electrophysiological methods to measure dendritic dynamics in the intact brain. |
|
Developing Novel In Vivo Imaging Techniques
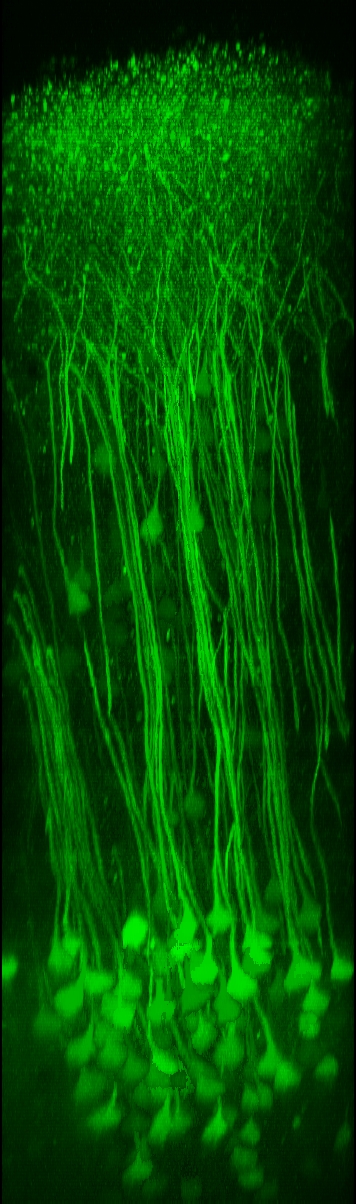
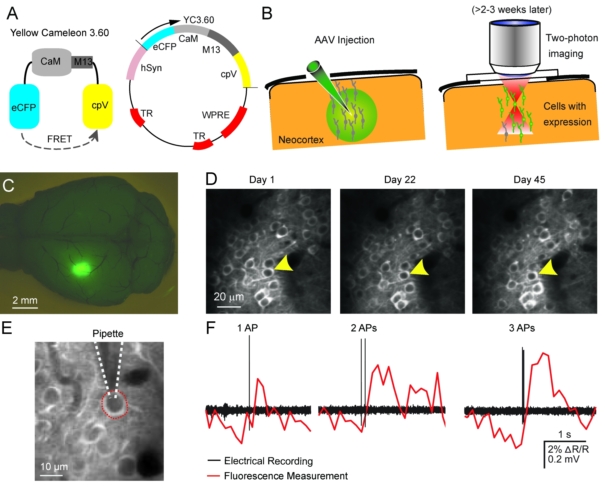
Two-photon calcium imaging techniquesTwo-photon laser-scanning microscopy has become the method of choice for imaging nerual dynamics on the cellular level in the intact brain. The particular advantage of 2-photon microscopy is its reduced sensitivity to light scattering so that individual cells with their dendrites can be resolved several hundreds of micrometers deep in neural tissue in living animals (Helmchen and Denk, 2005). Various fluorescence labeling techniques nowadays permit staining of individual cells and neuronal populations with great detail. To read out neuronal activity we apply fluorescence calcium indicators to the mouse brain. Powerful genetically-encoded calcium indicators such as GCaMPs, R-CaMPs (Bethge et al. 2017),and Yellow Cameleons (Lütcke et al. 2010; Chen et al., 2013; see Figure) are now available, which can be stably expressed in the mouse brain and thus in particular enable repeated activity measurements from the exact same cells over several months. |
|
Specialty two-photon imaging technologies
To enhance our capabilities to read out neuronal network dynamics using fluorescent indicators, we have developed advanced special laser-scanning modes that allow measurements in 3D (Göbel et al. 2007), at high speed (Grewe et al. 2010; Grewe et al. 2011) or from multiple fields across large areas at once (Chen, Voigt et al. 2016). These technological advances will help us to decipher the neural code in large, distributed networks of neurons for example in mouse neocortex.
Wide-field imaging approaches
In a complementary approach we take a zoomed-out view and measure brain dynamics at the large scale using sCMOS camera fluorescence imaging and novel transgenic mouse lines with specific expression of calcium indicators (Madisen et al. 2015; Bethge etal. 2017). Moreover, we apply fiber-photometry (Schukz et al. 2012), targeting thin optical fibers to specific brain regions, especially difficult to access subcortical areas, for bulk activity measurements. Our goal is a more comprehensive understanding of brain-wide signal flow during specific behavior such as the texture discrimination task.
Miniaturized microscopes
Over the past decades we have also contributed to the development of miniaturized two-photon microscopes using fiber optics, in order to enable high-resolution imaging of neuronal activity in freely behaving rodents (Helmchen et al. 2001; Engelbrecht et al. 2008)